Page 11 - Binder3
P. 11
Clinical Trials and Research
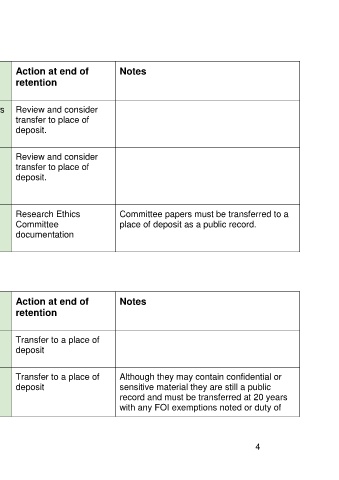
Record Type Retention starts Retention period Action at end of Notes
retention
Research data sets Closure of research Not more than 20 years Review and consider
transfer to place of
deposit.
Research Ethics End of research 5 years Review and consider
Committee transfer to place of
documentation for deposit.
research proposal
Research Ethics Year to which they Before 20 years Research Ethics Committee papers must be transferred to a
Committee minutes relate Committee place of deposit as a public record.
and papers documentation
Corporate Governance
Record Type Retention starts Retention period Action at end of Notes
retention
Board Meetings Creation Before 20 years/ as Transfer to a place of
soon as practical deposit
Board Meeting Creation Up to 20 years Transfer to a place of Although they may contain confidential or
(Closed Boards) deposit sensitive material they are still a public
record and must be transferred at 20 years
with any FOI exemptions noted or duty of
Delivering Quality Services – Information Governance Guidance 4